CISSPECT: using radiolabeled cisplatin in precision medicine
CISSPECT: using radiolabeled cisplatin in precision medicine
17 May 2024
Unique FIELD-LAB research collaboration
Being able to predict which cancer patient is likely to respond to chemotherapy with cisplatin has not yet been possible. However, a unique research project between NRG|PALLAS, the Netherlands Cancer Institute (NKI) and Amsterdam UMC may bring about some change. By administering 195mPlatinum radiolabeled cisplatin ([195mPt]cisplatin) to cancer patients, researchers aim to assess which patients will respond to therapy and who will not benefit and thus may not be given this treatment.
Subscribe to our newsletter!
Predict response to cisplatin
Although cisplatin has been used for decades in various types of cancer, its whereabouts in the human body are still not fully understood, says nuclear medicine specialist dr. Wouter Vogel (NKI).
Clinical pharmacologist prof. dr. Harry Hendrikse (Amsterdam UMC) adds: “Cisplatin is characterised by a highly varied uptake in the body; whether this correlates with efficacy is yet unknown.”
Vogel: “By making cisplatin radioactive, we can trace it in cancer patients and hopefully evaluate whether it will be effective. It may also provide more insights into adverse events, especially nephrotoxicity.”
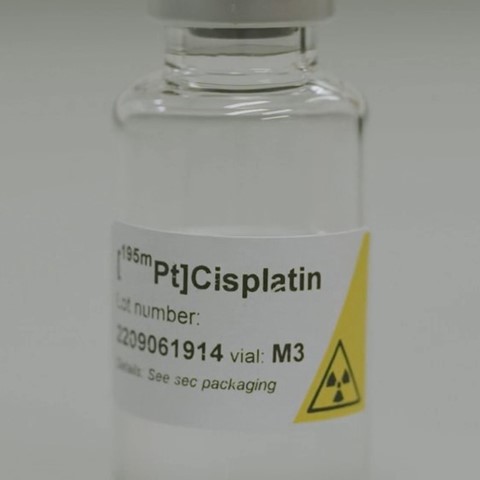
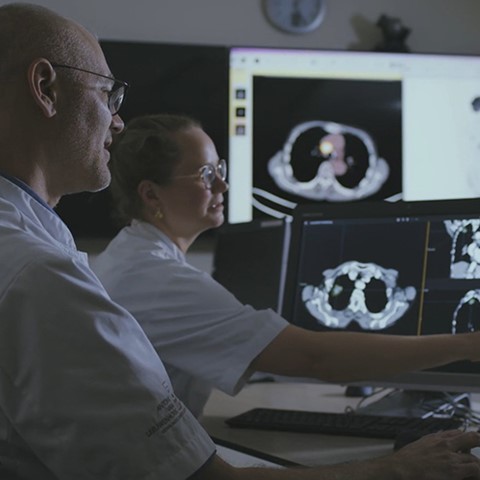
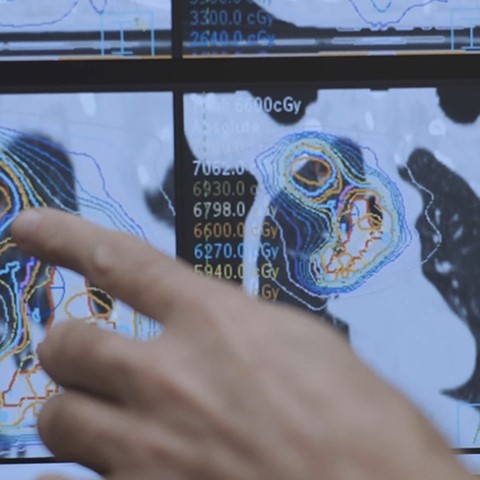
Vogel points out that adding cisplatin to radiotherapy increases the mean survival at 2 years by 5-10%, but with variations between individuals. “For many patients, cisplatin does not provide any benefit. However, this treatment with cisplatin may bring about (severe) adverse events. Therefore, it would be very useful being able to predict response as it means patients do not need to be subjected to ineffective yet potentially harmful therapy.”
This approach is part of an increasingly popular strategy which may help transform universal treatment (‘one size fits all’) into precision medicine, according to Hendrikse. “Precision medicine does not only offer a personal advantage for the patient, as he or she receives the best possible treatment as soon as possible, it may also have a significant pharmaco-economic advantage. It enables clinicians to use therapies, including the costly newer (targeted) oncology treatments, exclusively in those patients that will benefit from them.”
"This unique collaboration enables us to gather new insights which would not have been possible without the participation of NRG|PALLAS" - dr. Wouter Vogel, NKI
Extraordinary project partnership enables clinical pilot study
The first scientific research ‘Preclinical imaging characteristics and quantification of Platinum-195m SPECT’ was initiated by technical medical specialist dr. Else Aalbersberg (NKI).* It was to be the starting point of the clinical pilot study with [195mPt]cisplatin or CISSPECT®.
“We started preparations into this type of investigation almost 10 years ago together with NRG who developed CISSPECT®; for this particular study, it took 2 years to get ready”, Aalbersberg explains. “Organising the logistics for a study involving radioactive chemotherapy proved to be pretty complicated but it worked out well. The fact that the reactor, the laboratory where the radiopharmaceutical [195mPt]cisplatin is prepared according to good manufacturing practice (GMP) and the hospital where patients are treated, are located less than 100 kilometres away from each other is truly unique and certainly helps!”
The Netherlands Cancer Institute
Amsterdam, The Netherlands


Amsterdam UMC
Amsterdam, The Netherlands
Promising results
The first phase of the study is now well underway. A total of 5 lung cancer patients (of a total number of 6 to be included) have been administered [195mPt]cisplatin. The assumption is that if [195mPt]cisplatin does not seem to enter into the tumour – which is assessed by scans – it will probably have no efficacy. Patients do not need to stay in the hospital after administration of [195mPt]cisplatin; the scans are made during the time between chemotherapy and radiotherapy.
Aalbersberg: “We are quite happy with what we have seen so far; it looks promising. Colleagues in South-Africa have performed a similar type of study – albeit in healthy volunteers – and in due time, we would like to compare their and our results to see what we can learn from each other.”
Vogel emphasises the preliminary aspect of the research: “Our study may show a possible benefit, but as it is a clinical trial, it may emerge that [195mPt]cisplatin imaging does not have a benefit at all. We just have to wait and see. However, this unique collaboration enables us to gather new insights which would not have been possible without the participation of NRG|PALLAS.”
According to Hendrikse, NRG|PALLAS, NKI and Amsterdam UMC all need each other: “There is an enormous demand for products such as [195mPt]cisplatin within nuclear medicine: being able to deliver and trustworthiness are key elements. All of us involved are well-aware of the importance of this collaboration within the FIELD-LAB project, which hopefully translates into fruitful outcomes and further research in the years to come.”
Reference
* Aalbersberg EA, et al. Preclinical imaging characteristics and quantification of Platinum-195m SPECT. Eur J Nucl Med Mol Imaging. 2017;44:1347-1354.







